Introduction
Bentracimab is an innovative drug that has the potential to be a lifesaving treatment for patients taking Ticagrelor. Ticagrelor is a popular antiplatelet medication that helps prevent blood clots in people with heart conditions, reducing the risk of heart attacks and strokes. However, in emergency situations where surgery is needed, or a patient is experiencing uncontrolled bleeding, the antiplatelet effects of Ticagrelor need to be quickly reversed. This is where Bentracimab becomes crucial—it provides a fast-acting solution for reversing the effects of Ticagrelor. In this article, we’ll explore what Bentracimab is, how it works, and its potential impact on emergency cardiovascular care.
What is Bentracimab?
Bentracimab is a monoclonal antibody fragment designed to neutralize Ticagrelor and restore the body’s ability to form blood clots when necessary. Bentracimab binds directly to Ticagrelor, preventing it from blocking platelet function. This is typically how reversal agents act, by sequestering the active (or proactive form) drug. This makes it particularly useful in emergency situations such as urgent surgery or significant, uncontrolled bleeding.
Why is Bentracimab Needed?
Ticagrelor is an important drug for patients at high risk of heart attacks and strokes because it prevents platelets from sticking together and forming harmful clots. While this property is beneficial in preventing heart-related events, it also poses a risk of uncontrolled bleeding. Severe bleeding frequency falls somewhere in the 2-3% range. For patients who experience unexpected trauma or need emergency surgery, the inability to form clots can lead to life-threatening complications.
This is where Bentracimab comes in—by providing a rapid reversal of Ticagrelor’s effects, Bentracimab helps restore clotting ability, allowing healthcare providers to manage bleeding effectively or perform surgery safely. Having a dedicated reversal agent provides an extra layer of safety for patients on Ticagrelor, offering peace of mind for both patients and healthcare providers.
Is Bentracimab FDA Approved?
As of now, Bentracimab is under review by the FDA as part of a Biologics License Application (BLA). A BLA is a request for permission to introduce a biologic product, and it involves rigorous evaluation of the product’s safety and efficacy. Bentracimab has been granted Priority Review by the FDA, which indicates its potential significance in improving patient care. Often, unmet need or medical necessity are central to this urgency. The anticipated timeline for approval will be critical in determining when Bentracimab can be made available to patients who need it most.
Clinical Trial Results
Clinical trials have demonstrated that Bentracimab is highly effective in rapidly reversing the antiplatelet effects of Ticagrelor. Patients treated with Bentracimab experienced near-immediate restoration of platelet function, which is vital during emergencies. The trial results showed that the effects of Ticagrelor were reversed within minutes, allowing doctors to control bleeding and proceed with necessary interventions. This makes Bentracimab a promising tool for improving safety in patients taking Ticagrelor.
Safety Profile
Bentracimab has shown a favorable safety profile in clinical studies. The most common side effects reported were mild, such as injection site reactions and headache. There have been no significant safety concerns that would limit its use in emergencies, making Bentracimab a reliable option for reversing Ticagrelor. As with any medical intervention, patients should discuss the potential risks and benefits with their healthcare provider, but the data so far suggests that Bentracimab is both effective and safe for its intended use.
Conclusion
Bentracimab represents a significant advancement in the field of emergency cardiovascular care. By providing a fast and effective way to reverse Ticagrelor’s antiplatelet effects, Bentracimab could save lives in critical situations where bleeding control or urgent surgery is necessary. As we await FDA approval, healthcare providers are hopeful that Bentracimab will soon become an essential tool in managing cardiovascular emergencies.
As a takeaway, I like this chart below to demonstrate when the use of Bentracimab might be most clinically valuable. If the terminal action is “Neutralise APAs” then it would be best to use the reversal agent. Let’s await the prescribing information guidance when it comes to market.
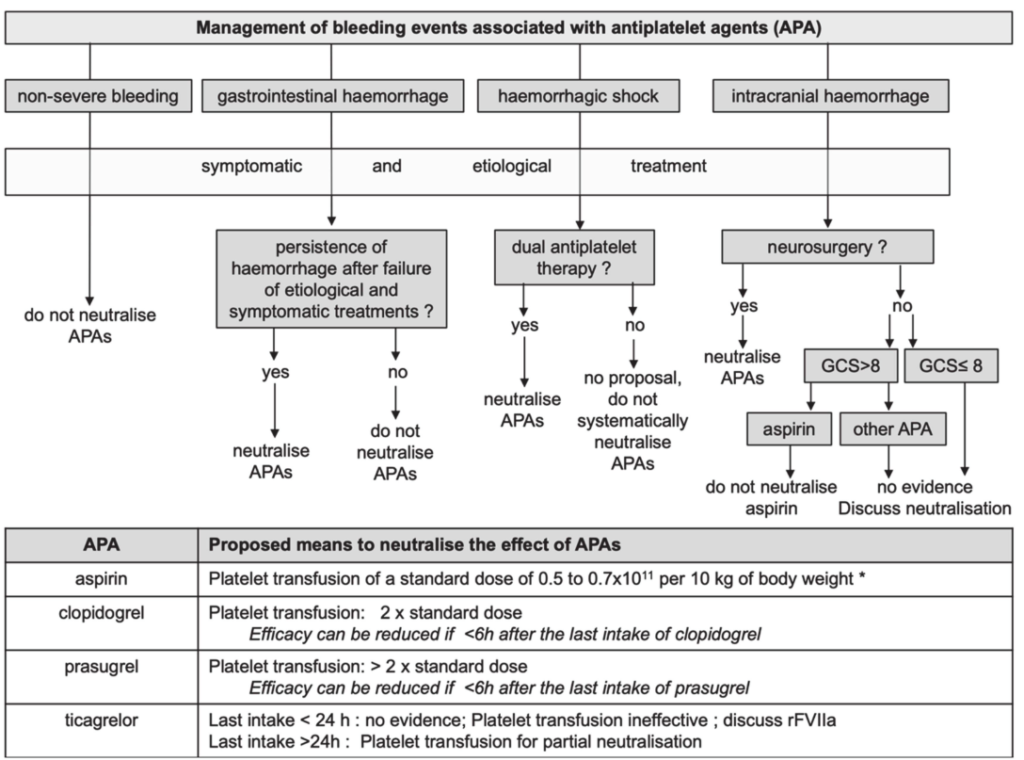
Credit: French Working Group on Perioperative Haemostasis (GIHP), 2020







