Why It Matters
Drug prior authorization (PA) policies sit at the intersection of evidence-based medicine, health plan resource management, and patient access in pharmacy. A simple drug prior authorization policy isn’t just a coverage filter—it’s a clinical tool that promotes appropriate use of drugs while minimizing confusion for prescribers, patients, and health plan reviewers. Simplicity is the key to wider awareness.
This Post Is For:
- Health plan and PBM decision-makers seeking to improve policy clarity
- Brokers and access consultants who need usable references
- Pharma and biotech specialists aligning approach for coverage optimization
- Clinicians navigating utilization management (UM) criteria
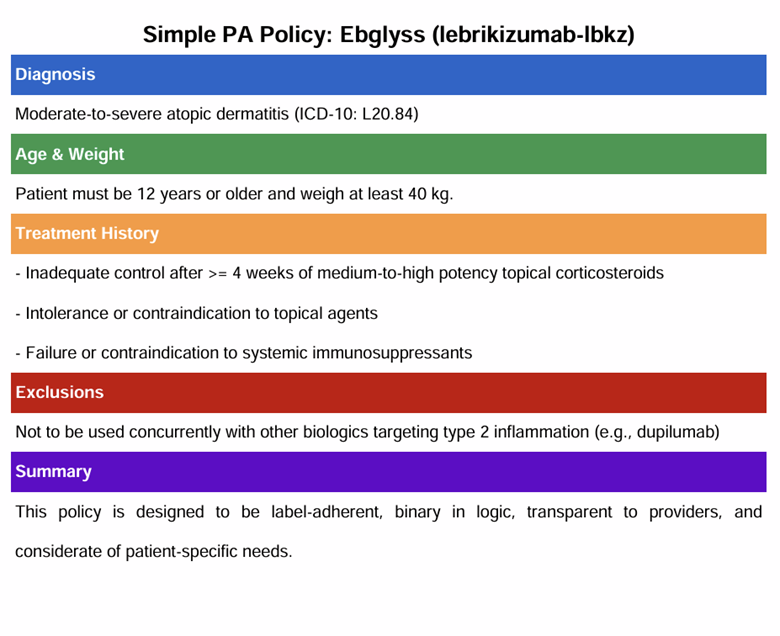
Example Drug: Ebglyss™ (lebrikizumab-lbkz)
Indication Label (Prescribing Information)
“Ebglyss is indicated for the treatment of adults and pediatric patients 12 years and older with moderate-to-severe atopic dermatitis who weigh at least 40 kg and whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.”

Building a Simple PA: Key Principles
1. Be Explicit with Criteria
A simple drug prior authorization policy avoids implied or overly interpretive language. It clearly states:
- Diagnosis and ICD-10 code
- Age and weight thresholds
- Required prior therapies, including duration or failure criteria
- Relevant labs or severity scores (with objective cutoffs)
- Route of administration (e.g., subcutaneous vs IV)
2. Align with Guidelines and the Label
Simple PAs should track closely to:
- FDA-approved prescribing information
- National clinical guidelines (e.g., AAD, GINA, ACR)
- Clinical consensus in evolving areas (real-world evidence, off-label use, label expansions)
Bonus: Consider flagging which criteria are linked to rebate eligibility to maximize transparency and value communication.
3. Keep It Human-Readable
Avoid unnecessary jargon. When complex terms (e.g., “interleukin-13 antagonist”) are required, provide brief definitions or context.
Goal: A frontline prescriber or pharmacist should be able to scan and understand in under 30 seconds.
4. Design for Portability
Use consistent formatting for ease of review. Enhance clarity with:
- Tagging (e.g., diagnosis, step therapy, duration)
- Color-coding by category
- Metadata: source, last review date, contact information
Bonus: What to Include in a Simple PA Footer
Include a compact metadata box for:
- Policy relevance (plan/formulary context)
- Last review or revision date
- Authorization duration (e.g., 6 months initial, 12 months renewal)
- Dynamic or live compliance tag (annual review)
- Internal strategy notes (e.g., monitored for step therapy fraud, waste or abuse, preferred tiering, formulary placement)